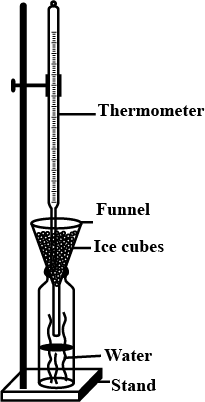
The melting point of ice in Fahrenheit is a crucial aspect of thermodynamics and the physical properties of water. The melting point is the temperature at which ice, a solid form of water, transitions into a liquid state. In this article, we will delve into the concept of the melting point of ice in Fahrenheit, explore its significance, and understand the factors that influence this critical temperature.
Understanding the Melting Point of Ice in Fahrenheit
The melting point of ice in Fahrenheit is precisely 32 degrees. At this temperature, ice undergoes a phase change, transitioning from a solid to a liquid. This value is well-known and often used as a reference point for temperature scales, particularly in countries that utilize the Fahrenheit system of measurement.
Significance of the Melting Point of Ice
The melting point of ice in Fahrenheit holds great significance in various fields, such as physics, chemistry, and engineering. It serves as a fundamental benchmark for temperature measurements and provides a baseline for the calibration of thermometers. Additionally, understanding the melting point of ice is crucial in various applications, such as refrigeration, cryogenics, and the study of climate change.
Factors Affecting the Melting Point of Ice
Several factors can influence the melting point of ice in Fahrenheit. One crucial factor is pressure. Ice melts at a lower temperature as pressure rises. This principle explains why ice skating is possible—the pressure exerted by the skater’s weight lowers the melting point of the ice, allowing it to temporarily melt and facilitate smoother movement.
Impurities in water can also affect the melting point of ice. Pure water freezes and melts at 32 degrees Fahrenheit. However, when impurities are present, such as dissolved salts or minerals, the melting point can be lower or higher than the standard 32 degrees. These impurities disrupt the crystal structure of the ice, affecting the energy required for the phase transition.
Applications and Practical Implications
Understanding the melting point of ice in Fahrenheit is crucial in various practical applications. For instance, in refrigeration systems, knowledge of the melting point allows engineers to design and optimize cooling processes. By manipulating pressure and controlling impurities, refrigeration systems can efficiently maintain temperatures below the melting point, preserving perishable goods and providing comfortable environments.
Furthermore, the melting point of ice in Fahrenheit is a vital parameter in climate change studies. Scientists monitor the temperature at which glaciers and ice sheets melt to assess the impact of global warming. As temperatures rise, the melting point is exceeded more frequently, leading to the loss of ice mass and contributing to sea level rise.
Conclusion
The melting point of ice in Fahrenheit, at precisely 32 degrees, is a critical temperature in our understanding of water’s physical properties. It serves as a reference point for temperature measurement and finds applications in various scientific and practical fields. Factors such as pressure and impurities can affect the melting point, showcasing the complex nature of this phenomenon. As we continue to explore the intricate behavior of water and its phase transitions, the knowledge of the melting point of ice remains a cornerstone in our understanding of the natural world.